TMS Therapy in Staunton, VA
A New Alternative to Antidepressant Medications
Transcranial magnetic stimulation (TMS) is a non-invasive procedure that uses magnetic fields to stimulate brain cells to improve symptoms of depression, OCD, and other disorders. TMS uses magnetic pulses to induce small electrical currents in the brain cortex. These magnetic pulses occur at a specific frequency that either stimulates or inhibits the brain region they target. TMS has been typically used when other treatments haven’t been effective. TMS Therapy is FDA approved for treatment-resistant depression and Obsessive Compulsive Disorder and may also aid in the treatment of anxiety, PTSD, Tourette’s, challenges frequently experienced by ADHD and Autistic people, and chronic pain, among other uses.
A Non-Invasive Depression Treatment
A New Depression Treatment
NeuroStar TMS Therapy is a new treatment cleared by the US Food and Drug Administration (FDA) for patients suffering from depression who have not achieved satisfactory improvement from prior antidepressant treatment.
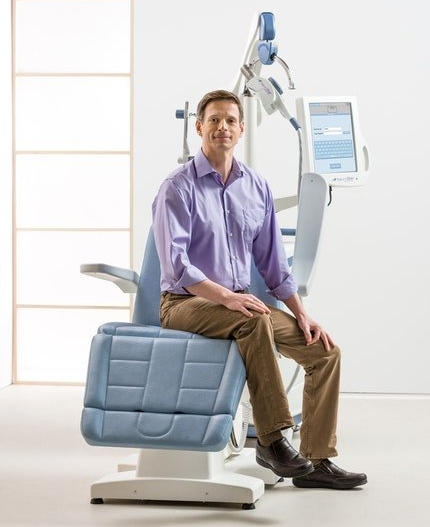
Transcranial Magnetic Stimulation
TMS stands for "transcranial magnetic stimulation”. TMS Therapy is a treatment that can be performed in a psychiatrist's office, under their supervision, using a medical device called the NeuroStar TMS Therapy system.
Non-Invasive
TMS Therapy is a non-invasive depression treatment, meaning that it does not involve surgery. It does not require any anesthesia or sedation, as the patient remains awake and alert during the treatment.
Treatment Plan
The typical initial treatment course consists of at least 5 treatments per week over a 4-6 week period, for an average of 20-30 total treatments. Each treatment session lasts approximately 19-40 minutes, depending on what the doctor determines is the correct protocol.